Classification of Hydrocarbons
Classification of Hydrocarbons: Overview
This topic covers concepts, such as, Degree of Carbon Atoms etc.
Important Questions on Classification of Hydrocarbons
Identify the primary halide among the following compounds.
Which of the following alkanes contain primary, secondary, tertiary and quaternary carbon atoms together?
The number of and atoms in -ethyl-- methyl heptane, respectively, is:
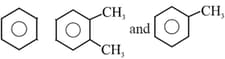
Number of secondary carbon atoms present in the compounds is respectively:
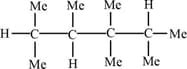
Number of carbon and hydrogen respectively in the following structure are -
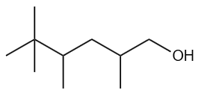
Which of the following compounds contains maximum number of tertiary hydrogen atoms?
The smallest hydrocarbon which contains secondary carbons only is -
The smallest hydrocarbon which contains secondary carbons only is:
The smallest hydrocarbon which contains secondary carbons only is -
The number of tertiary atoms in tetramethyl pentane is
The number of secondary hydrogens in 2, 2-dimethyl butane is
2, 3-dimethyl hexane contains ..... tertiary ..... secondary and ......primary carbon atmos, respectively
The number of primary, secondary and tertiary hydrogens in the following compound are, respectively
Match the compounds in column I with their structure(s)/ characteristic(s)/test(s)/reaction(s)/stereochemistry,etc., given in column II. Matching can be one or more than one.
| Column I | Column II | ||
|---|---|---|---|
| S.No. | Compound | Nature of H atoms | |
| a. | 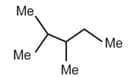 |
p. | 15(1o H), 4(2oH), 1(3oH) |
| b. | 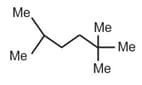 |
q. | 17(1o H), 2(2o H), 2(3o H) |
| c. | 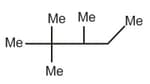 |
r. | 12(1o H), 2(2oH), 2o(3o H) |
| d. | 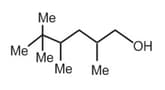 |
s | 15(1o H), 2(2o H), 1o(3o H) |
Match the compounds in column I with their structure(s)/ characteristic(s)/test(s)/reaction(s)/stereochemistry,etc., given in column II. Matching can be one or more than one.
| Column I | Column II | ||
|---|---|---|---|
| S. No. | Compound | Structure | |
| a. | C8H18 with only 1o H atoms | p. | 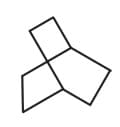 |
| b. | C6H12 with only 2o H atoms | q. | 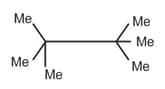 |
| c. | C6H12 with only 1o and 2o H atoms | r. | 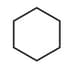 |
| d. | C8H14 with 12 secondary and 2 tertiary H atoms | s. | 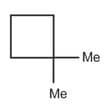 |
